Courses


 Compare
Compare
Heat: It is a form of energy which transfers among particles in a substance (or system) by means of kinetic energy of those particles. In other words, a form of energy associated with the motion of atoms or molecules and capable of being transmitted through solid and fluid media by conduction, through fluid media
0 Lessons
Hours
 Compare
Compare
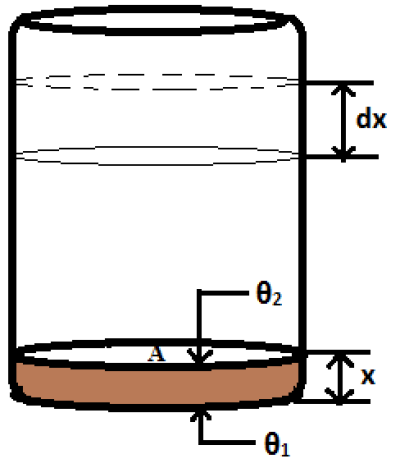
Let a long metal bar of uniform cross-section is heated steadily at one end M and the bar is so long that the other end N may be regarded to be at the same temperature as that of the surroundings (Fig. 8). Now we shall consider two neighboring planes at P and Q at distances x and x+δx from the hot end, therefore we have considered a layer PQ. Let θ be the excess temperature of the layer at P over the surroundings,
0 Lessons
Hours
 Compare
Compare
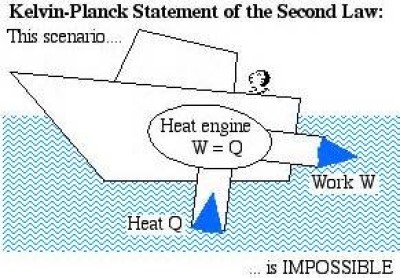
Thermodynamics: In heat engine there was an input in the form of heat whose output was mechanical work (Fig. 10). This predicted the principle of thermodynamics. Therefore, thermodynamics was concerned with both thermal and mechanical or dynamical concepts
0 Lessons
Hours
 Compare
Compare
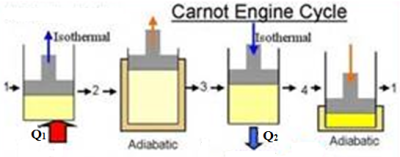
A cycle in which the working substance starting from a given condition of temperature, pressure and volume is made to undergo two successive expansions (one isothermal and another adiabatic), and then two successive compressions (one isothermal and another adiabatic) at the end of which the working substance is brought back
0 Lessons
Hours
 Compare
Compare
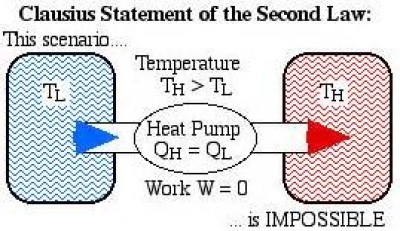
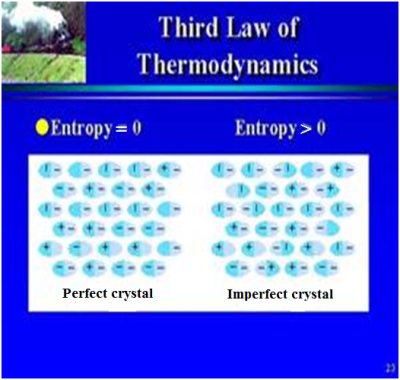
2nd law of thermodynamics state that it is not possible for heat to flow from a colder body to a warmer body without any work having been done to accomplish this flow
0 Lessons
Hours
 Compare
Compare
Maxwell derived the relations by combining the first and the second law of thermodynamics. For a P, V and T system undergoing an infinitesimal reversible process, we have from the first law of thermodynamics
0 Lessons
Hours
 Compare
Compare
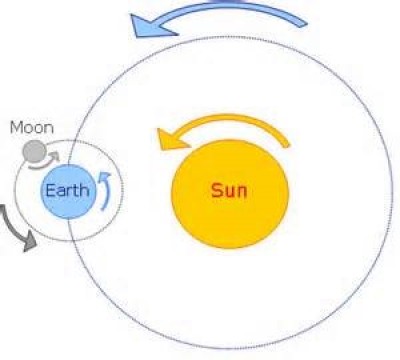
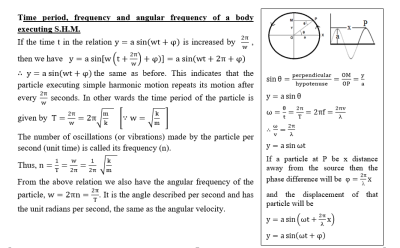
A motion which repeats itself over and over again after a regular interval of time is referred to as a periodic motion. The motion of moon about the earth, the oscillation of a pendulum, the motion of a mass suspended from a coil spring are the examples of periodic motion (Fig. 1).
0 Lessons
Hours
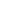 Compare
Compare
Many objects on the microscopic level, such as molecules, atoms, nuclei, execute oscillations that are approximately simple harmonic. Let us consider a diatomic molecule, in which the two atoms are bound together with a force and if displaced in a small distance from its equilibrium position,
0 Lessons
Hours
 Compare
Compare
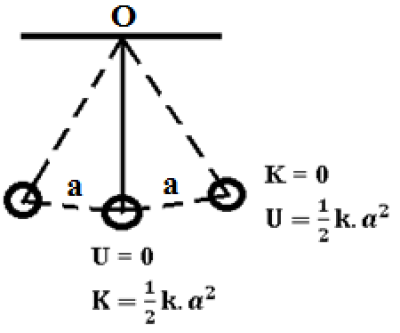
The mechanical energy E of a particle executing simple harmonic motion is partly kinetic and partly potential. If no non-conservative forces, such as the force of friction act on the particle, the sum of its kinetic energy (K) and potential energy (U) remains constant. Therefore, total energy, E = K + U = constant.
0 Lessons
Hours