Course description
Entropy:
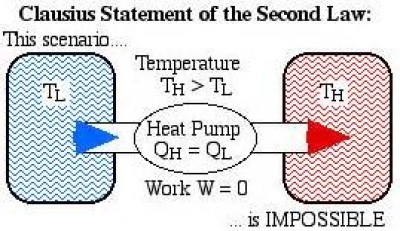
2nd law of thermodynamics state that it is not possible for heat to flow from a colder body to a warmer body without any work having been done to accomplish this flow (Fig. 25 a). This implies that heat naturally flows in the direction of decreasing temperature, i.e., identifies the direction of the process. In other words, heat can only spontaneously transfer from a hot object to cold object, not vice versa (Fig. 25 b). It also implies that energies always flow to equilibrate and introduces the concepts of reversible and irreversible process.
(a)
For example, molecules move (diffuse) from an area of high concentration to areas of low concentration and eventually, molecules become randomly distributed unless acted on by something else (Fig. 25 c). However, spontaneous processes in nature always proceed in such a way that the disorder of the universe increases (Fig. 25)
(b)
The quantitative measure of the disorder in a system is known as entropy and there are no negative values of entropy. A highly ordered system has low entropy. The law of disorder states the natural tendency is for systems to move to the direction of maximum disorder, not vice-versa.
(c)
An increase in entropy favors the spontaneous chemical reaction. A decrease in entropy favors the non-spontaneous reaction. Therefore, all things tend toward entropy (randomness). Moreover, in any natural process, there exists an inherent tendency towards the dissipation of useful energy.(d)
Clausius described entropy as the transformation-content, i.e. dissipative energy use, of a thermodynamic system or working body of chemical species during a change of state.
The entropy of the system is measured in terms of the changes the system has undergone from the previous state to the final state.
(e)
Thus, the entropy is always measured as the change in entropy of the system denoted by ∆S and not merely S.
If a system receive or gives out a quantity of heat ∆Q at a temperature T K then the change in entropy of the system, ∆S=∆Q/T .
(f)
Therefore, entropy is the extensive property of the system (depends on the mass of the system) and its unit of measurement is J/K (Joule per degree Kelvin).
Moreover, entropy is heat or energy change per degree Kelvin temperature. Entropy is nothing but the unavailability of energy.
The entropy of a body in all reversible processes remains constant (total change in entropy is zero), but in the irreversible cycle there is a net increase in entropy (Fig. 25 j)
(g)