Course description
Thermodynamics: In heat engine there was an input in the form of heat whose output was mechanical work (Fig. 10). This predicted the principle of thermodynamics. Therefore, thermodynamics was concerned with both thermal and mechanical or dynamical concepts . Today scientists and engineers use the principle of thermodynamics in the design of internal combusting engines, refrigeration and air-conditioning systems, conventional and nuclear power stations, and propulsion systems for aircrafts, rockets, missiles, ship and land vehicles. Thus it may say that thermodynamics deals with the transformation of heat energy to other forms of energy, such as mechanical, electrical, magnetic, and radiant, etc.
Fig 10
The zeroth law of thermodynamics:
It states that when any two bodies are each separately in thermal equilibrium with a third, then they are also in thermal equilibrium with each other (Fig. 11).
First law of thermodynamics:
According to this law, a definite amount of heat is needed to do a definite amount of mechanical work and vice versa. If Q and W be the amount of heat and work done, then
W = JQ --------------------------(i)
where J is a constant called the mechanical equivalent of heat.
From (i) J=W/Q ; if Q = 1 cal then J = W.
It is known that one calorie of heat is needed to be done 4.186 joules of work somehow. Therefore, J = 4.186 joules per calorie = 4186 joules per kilocalorie.
Moreover, this law implies that the amount of heat supplied to a system must be balanced by the sum of the gain in internal energy of the system and the external work done (Fig. 12). Therefore, ∆Q = ∆U + ∆W
where ∆Q = heat supplied, ∆U = change in internal energy and ∆W = external work done. This law however, an extension of the law of conservation of energy.
Reversible and irreversible processes:
When a system undergoes a change from its initial state to the final state, one or more of the thermodynamics properties of the system like temperature, pressure, volume, enthalpy or heat, entropy, etc. changes.
Reversible process: During the thermodynamic process, if the system and surroundings can be restored to the initial state from the final state without producing any changes in the thermodynamics properties of the system is called a reversible process. Therefore, the reversible process can be reversed completely and there is no trace left to show that the system had undergone thermodynamic change.
For example, let us consider a mixture of water and ice at 00C in a vessel in which the temperature and heat exchange can be controlled as required. When the mixture is cooled so that its temperature falls by the slightest amount, then water will freeze and its volume will increase (Fig. 14).
If the mixture is now heated so that its temperature rises by the same slightest amount, then the ice will melt and so decrease in volume (Fig. 14). Hence, the changes of volume is reversed as the substance freezes or melts. It can be said that the process takes place at practically constant temperature and considered as isothermal process.
Moreover, the reversible process is possible if the changes in pressure and volume of the working substance must take place at an extremely slow rate and no friction and no loss of heat are occurred by conduction, convection and radiation. Therefore, during reversible process all the changes in state that occur in the system are in thermodynamic equilibrium with each other. However, this is not a spontaneous process.
Irreversible process: In an irreversible process, the system with the surroundings can never be completely restored to its initial condition by reversing the controlling factors.
All the changes, which occur suddenly like explosions, heat produced by friction or during the current flows through an electrical resistance, sudden unbalanced expansion and all the natural processes are the examples of irreversible process (Figs 15-18).
Therefore, this is a spontaneous process and no changes in state that occur in the system are in thermodynamic equilibrium with each other.
Second law of thermodynamics:
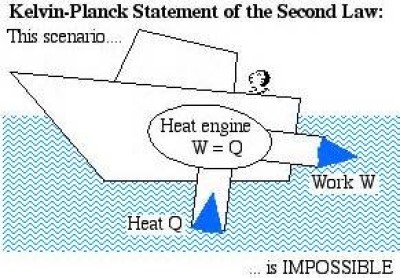
Kelvin-Plank statement: It is impossible to construct an engine, which, operating in a cycle, will produce no other effect than the extraction of heat from a reservoir and the performance of an equivalent amount of work (Fig. 19).
Fig 19
Clausius statement: It is not possible to construct a device, which operates in a cycle. While operating, it does not produce any effect other than transferring the heat from cooler to hotter body (Fig. 20).
In other words, it is not possible for heat to flow from a colder body to a warmer body without any work having been done to accomplish this flow.
Energy will not flow spontaneously from a low temperature object to a higher temperature object. Therefore, heat cannot spontaneously flow from cold regions to hot regions without external work being performed on the system, which is evident from ordinary experience of refrigeration, for example.
In a refrigerator, heat flows from cold to hot, but only when forced by an external agent, the refrigeration system (Fig. 21).
Fig 21