Course description
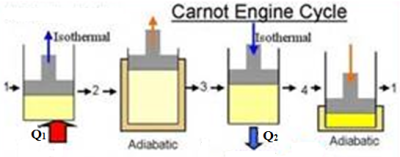
A cycle in which the working substance starting from a given condition of temperature, pressure and volume is made to undergo two successive expansions (one isothermal and another adiabatic), and then two successive compressions (one isothermal and another adiabatic) at the end of which the working substance is brought back to its initial condition, is called Carnot’s cycle (Fig. 23 a).
The essentials of any heat engine proposed by Carnot, are- (i) a cylinder with a piston, (ii) a source, (iii) a sink and (iv) a non-conducting stand as shown in Fig. 23 (b).
Let us now consider the four stages of a Carnot cycle. The original condition is presented by the point A (P1, V1, T1) on the P-V diagram (Fig. 23 c), where P1, V1 and T1 are pressure, volume and temperature respectively of the working substance like air.
Operation I (isothermal expansion): Let the bottom of the cylinder be placed in contact with the source and the gas is allowed to expand slowly. The fall in temperature during expansion is compensated by absorption of heat from the source. So the gas expands isothermally to the point B represented by P2, V2 and T1. During the process an amount of heat energy Q1 is absorbed from the source at constant temperature. This heat energy is equivalent to the amount of external work done by the gas, W1.
Where W1 = area ABV2V1.
Operation II (adiabatic expansion): The cylinder is removed from the source and its bottom is placed in contact with the non-conducting stand. The gas is allowed to expand. As the gas is completely isolated from the surroundings no heat enters or leaves the system during expansion. The process is, therefore, adiabatic and the temperature of the system falls to that of the sink (T2). Therefore, at the end of the adiabatic expansion the gas gets the point C (P3, V3, T2). The work done at this stage, W2 = area BCV3V2.
Operation III (isothermal compression): To bring the gas back to the initial conditions, the cylinder is removed from the non-conducting stand and its bottom is placed in contact with the sink at T2K. The piston is then pressed very slowly and the gas is compressed. The heat generated is given out to the sink and the compression, therefore, takes place isothermally at the constant temperature T2K and gets the point D (P4, V4, T2). During the process work is done on the gas, W3 = area CDV4V3.
Fig 23
The net work (W) done by the system during the cycle is given by
W = W1 + W2 - W3 - W4
W = W1 - W3 (since W2 = W4 )
W = area ABCD
Operation IV (adiabatic compression): The cylinder is now removed from the sink and its bottom is placed in contact with the insulating stand. The gas is compressed adiabatically until its temperature rises to T1K and its pressure and volume become P1 and V1, represented by the point A. The work done, W4 = area DAV1V4.
Carnot’s Theorem: The second law of thermodynamics leads to two important deductions known as Carnot’s theorem and these are:
(i) All reversible engines working between the same temperature limits have the same efficiency.
(ii) Working between the same initial and final temperatures, no engine can be more efficient that a reversible one (Fig. 24 a).
Second law of thermodynamics: It is not possible for heat to flow from a colder body to a warmer body without any work having been done to accomplish this flow.
A Carnot heat engine is an engine that operates on the reversible Carnot cycle (Fig. 24 b).
In an irreversible process, finite changes are made; therefore, the system is not at equilibrium throughout the process. At the same point in an irreversible cycle, the system will be in the same state, but the surroundings are permanently changed after each