Course description
Eqn. (i) expresses, the heat absorbed per unit volume in isothermal expansion is T times the rate of increase of pressure with temperature in an isochoric process.
Let us apply eqn. (i) to the equilibrium between two states of the same substance, say phase transition such as vapourisation of a liquid or melting of a solid. Now let a liquid in equilibrium with its vapour contained in a cylinder. The pressure is the saturated vapour pressure, which is a function of temperature only and is independent of the amount of liquid and vapour present. If the system be allowed to expand at constant temperature, the vapour pressure will remain constant; only some additional liquid, say of mass dm, will evaporate to fill the extra space with vapour. Then ∂Q=L .dm
∂V=(v_Vap-v_liq)dm ; where, L is the latent heat of evaporation per unit mass.
The corresponding volume change will be
; where, being the specific volumes of the vapour and the liquid respectively. [Specific volume = Volume/mass]
Thermodynamic potentials functions: There are four thermodynamic potentials or characteristic functions, which are useful in ascertaining the state of stable thermodynamic equilibrium for reversible processes. These are U (S, V), F (T, V), G (T, P) and H (S, P); where U = internal energy, S = entropy, V = volume, F = Helmholtz free energy, T = temperature, G = Gibbs’ free energy, P = pressure and H = enthalpy.
Third law of thermodynamics:
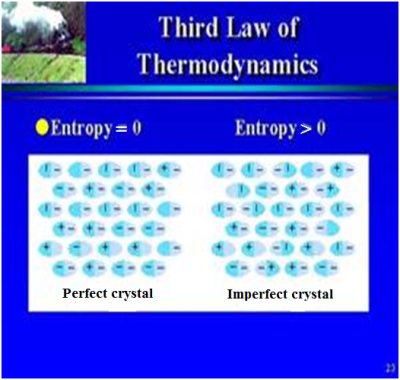
The 3rd law was developed by the chemist Walther Nernst and is therefore often referred to as Nernst's theorem or Nernst's postulate. The third law of thermodynamics states that the entropy of a system at absolute zero is a well-defined constant (this constant is zero) (Fig. 26). This is because a system at zero temperature exists in its ground state, so that its entropy is determined only by the degeneracy of the ground state. Absolute zero (0° Kelvin) corresponds to about -273.150Celsius, or -459.7 Fahrenheit.
An alternative version of the third law of thermodynamics as stated by Gilbert N. Lewis and Merle Randall: If the entropy of each element in some (perfect) crystalline state be taken as zero at the absolute zero of temperature, every substance has a finite positive entropy; but at the absolute zero of temperature the entropy may become zero, and does so become in the case of perfect crystalline substances.
This version states not only ΔS will reach zero at 0 K, but S itself will also reach zero as long as the crystal has a ground state with only one configuration.
In its shortest form, the Third Law of Thermodynamics says: The entropy of a perfect crystal, at absolute zero (zero Kelvin), is exactly equal to zero.
At zero Kelvin, the system must be in a state with the minimum possible energy, and this statement of the third law holds true if the perfect crystal has only one minimum energy state.
The Third Law of Thermodynamics can be visualized by thinking about water. Water in gaseous form has molecules that can move around very freely. Water vapor has very high entropy (randomness). As the gas cools, it becomes liquid. The liquid water molecules can still move around, but not as freely. They have lost some entropy. When the water cools further, it becomes solid ice. The solid water molecules can no longer move freely, but can only vibrate within the ice crystals. The entropy is now very low. As the water is cooled more, closer and closer to absolute zero, the vibration of the molecules diminishes. If the solid water reached at absolute zero, all molecular motion would stop completely. At this point, the water would have no entropy (randomness) at all.
In actuality, no object or system can have a temperature of zero Kelvin, because of the Second Law of Thermodynamics. The Second Law, in part, implies that heat can never spontaneously move from a colder body to a hotter body. So, as a system approaches absolute zero, it will eventually have to draw energy from whatever systems are nearby. If it draws energy, it can never obtain absolute zero. So, this state is not physically possible, but is a mathematical limit of the universe.
Fig 26
Heat and Thermodynamics
Q1. Define thermal conductivity of a material and write its SI unit.
Q2. Explain the thermal conductivity of the materials as given in the table.
Q3. Define temperature gradient.
Q4. Derive the standard (Fourier) equation for one-dimensional flow of heat in the variable / steady state.
Q5. Discuss the significance of thermodynamics.
Q6. First law of thermodynamics is an extension of the law of conservation of energy. Explain.
Q7. Distinguish between reversible and irreversible processes.
Q8. State and explain the second law of thermodynamics.
Q9. What do you mean by efficiency of a heat engine?
Q10. Describe Carnot cycle. Explain the each operation of the four stages of the Carnot cycle with the help of a P-V diagram.
Q11. Obtain an expression for the work done in each operation of the cycle and the net work done in the cycle.
Q12. State Carnot’s theorem.
Q13. Entropy of a substance is a measure of its state of disorder- discuss fully.
Q14. 2nd law of thermodynamics implies the entropy. Explain.
Q15. Derive Maxwell’s thermodynamic relations.
Q16. Derive Clausius-Clapeyron’s latent heat equation.
Q17. What are the thermodynamic functions? Give explanation.
Q18. State third law of thermodynamics, give example. Why this statement is not physically possible?