The Mighty Atom
The physicist Richard Feynman once said that if you had to reduce scientific history to one statement it would be “All things are made of atoms.”
English
Last updated
Sun, 30-Jun-2024
The Mighty Atom
The physicist Richard Feynman once said that if you had to reduce scientific history to one statement it would be “All things are made of atoms.”
 Atoms group together in molecules. A molecule is just two or more atoms in a more or less stable arrangement: add two atoms of hydrogen to one of oxygen and you have a molecule of water. At sea level, at a temperature of 32 degrees Fahrenheit, one cubic centimeter of air (that is, a space about the size of a sugar cube) will contain 45 billion billion molecules.
Atoms group together in molecules. A molecule is just two or more atoms in a more or less stable arrangement: add two atoms of hydrogen to one of oxygen and you have a molecule of water. At sea level, at a temperature of 32 degrees Fahrenheit, one cubic centimeter of air (that is, a space about the size of a sugar cube) will contain 45 billion billion molecules.
They also last a very long time and they seem to like new experiences. Every atom in you has passed through several stars and been part of millions of organisms on its way to becoming you. We are so much recycled at death that a large number of our atoms came from Genghis Khan and Aurangzeb, historical figures as it takes atoms decades to be redistributed. So, for better or worse, you are not yet one with ex-President Ershad!
When we die our atoms will move off to find new uses elsewhere – as part of a leaf or other human being. Atoms, however, go on forever. Nobody actually knows how long an atom can survive, but it is probably about 1035years.
Above all, atoms are very tiny indeed. Half a million of them could hide together behind a human hair. Start with a millimeter, which is a line this long: -. Now imagine that line divided into a thousand equal widths. Each of those widths is a micron. That is the size of microorganisms. However, atoms exist on a different scale of minuteness altogether though. You would need to take each of those micron slices and shave it into ten thousand finer widths. That’s the scale of an atom: one ten-millionth of a millimeter.
 The realization that atoms are these three things – small, countless, impossible to destroy – and that all things are made from them first occurred to a not very well-educated Englishman named John Dalton. Dalton was born in 1766 to a family of poor Christians. He was an exceptionally bright student – so bright that at the age of twelve he was put in charge of the local school. We know from his diaries that at about this time he was reading Newton’s Principia in the original Latin. In Manchester at the age of 25, he was producing books and papers on subjects from meteorology to grammar. But it was A New System of Chemical Philosophy, published in 1808, that made his reputation.
The realization that atoms are these three things – small, countless, impossible to destroy – and that all things are made from them first occurred to a not very well-educated Englishman named John Dalton. Dalton was born in 1766 to a family of poor Christians. He was an exceptionally bright student – so bright that at the age of twelve he was put in charge of the local school. We know from his diaries that at about this time he was reading Newton’s Principia in the original Latin. In Manchester at the age of 25, he was producing books and papers on subjects from meteorology to grammar. But it was A New System of Chemical Philosophy, published in 1808, that made his reputation.
There, in a chapter of just five pages (out of more than nine hundred), we first met atoms. Dalton’s insight was that at the root of all matter are exceedingly tiny, unchangeable particles. “We might as well try to introduce a new planet into the solar system as to create or destroy a particle of hydrogen,” he wrote.
Neither the idea of atoms nor the word itself was new. Both were developed by the ancient Greeks. Dalton considered the relative sizes and characters of these atoms and how they fit together. He knew, for instance, that hydrogen was the lightest element, so he gave it an atomic weight of one. He believed water consisted of seven parts of oxygen to one of hydrogen, and so he gave oxygen an atomic weight of seven. In this way, he arrived at the relative weights of the known elements. He wasn’t always accurate – oxygen’s atomic weight is actually sixteen, not seven – but the principle was the basis of modern chemistry and most of the rest of modern science.
Although Dalton tried to avoid honours: he was elected to the Royal Society against his wishes, showered with medals, and given a government pension. When he died in 1844, forty thousand people went to see his corpse and the funeral procession was two miles long.
For a century after Dalton suggested the idea of atoms, this stayed theoretical though. Some great scientists – like Ernst Mach, who named the speed of sound – doubted the existence of atoms at all. “Atoms cannot be observed with the senses . . . they are things of thought,” he wrote. It was Einstein who provided the first sure evidence of atoms with a paper in 1905, but this attracted little attention and, anyway, Einstein was soon to be interested only in his work on relativity. So the first real hero of the atomic age was Ernest Rutherford.
 Rutherford was born in 1871 in New Zealand to Scottish parents. He was about as far from the mainstream of science as possible, but in 1895 he won a scholarship to the Cavendish Laboratory at Cambridge University, which was about to become the hottest place in the world for physics. “All science is either physics or stamp collecting,” he once said. It is funny, therefore, that when he won the Nobel Prize in 1908, it was in chemistry, not physics. Rutherford was lucky to live at a time when physics and chemistry were so exciting.
Rutherford was born in 1871 in New Zealand to Scottish parents. He was about as far from the mainstream of science as possible, but in 1895 he won a scholarship to the Cavendish Laboratory at Cambridge University, which was about to become the hottest place in the world for physics. “All science is either physics or stamp collecting,” he once said. It is funny, therefore, that when he won the Nobel Prize in 1908, it was in chemistry, not physics. Rutherford was lucky to live at a time when physics and chemistry were so exciting.
For all his success, Rutherford was actually pretty terrible at mathematics. Often during lectures he would get so lost in his own equations that he would give up and tell the students to work it out for themselves. According to his colleague James Chadwick, discoverer of the neutron, he wasn’t even clever at experimentation. He was just hard-working and open-minded. His mind, in the words of one biographer, was “always operating as far as he could see, and that was a great deal further than most other men.” He was prepared to work at problems harder and longer. His breakthrough came because he spent boring hours counting alpha particle scintillations, as they were known – the sort of work that an assistant would normally do. He was one of the first to see that the power in the atom could make bombs powerful enough to “make this old world vanish in smoke.”
Physically he was big with a huge voice. Once when Rutherford was going to make a radio broadcast across the Atlantic, a colleague asked: “Why use radio?” He also had great confidence.
It was an especially busy period in science. In the year of his arrival in Cambridge, Wilhelm Roentgen discovered X rays in Germany, and the next year Henri Becquerel found radioactivity in France. And in 1897, J. J. Thomson and colleagues would discover the electron.
Rutherford worked on radio waves – he managed to transmit a signal more than a mile – but gave it up when he was persuaded that radio had little future. He received his Nobel Prize (for “investigations into the disintegration of the elements, and the chemistry of radioactive substances”).
By the early twentieth century it was known that atoms were made of parts – Thomson’s discovery of the electron had established that—but it wasn’t known how many parts there were or how they fitted together or what shape they took. Some physicists thought atoms might be cube-shaped, because cubes can be packed together without any wasted space. The more general view, however, was that an atom was a dense, solid object that carried a positive charge but that was covered with negatively charged electrons.
 In 1910, Rutherford fired ionized helium atoms, or alpha particles, at a sheet of gold foil. To Rutherford’s astonishment, some of the particles bounced back. He said it was like he had fired a fifteen-inch shell at a sheet of paper and it fell back into his lap. This should not happen. He realized there could be only one possible explanation: the particles that bounced back were striking something small and dense at the heart of the atom, while the other particles went through. An atom, Rutherford realized, was mostly empty space, with a very dense nucleus at the center. This was a wonderful discovery, but it made one immediate problem. By all the laws of physics, atoms shouldn’t therefore exist.
In 1910, Rutherford fired ionized helium atoms, or alpha particles, at a sheet of gold foil. To Rutherford’s astonishment, some of the particles bounced back. He said it was like he had fired a fifteen-inch shell at a sheet of paper and it fell back into his lap. This should not happen. He realized there could be only one possible explanation: the particles that bounced back were striking something small and dense at the heart of the atom, while the other particles went through. An atom, Rutherford realized, was mostly empty space, with a very dense nucleus at the center. This was a wonderful discovery, but it made one immediate problem. By all the laws of physics, atoms shouldn’t therefore exist.
Let us pause for a moment and consider the structure of the atom as we know it now. Every atom is made from three kinds of particles: protons, which have a positive electrical charge; electrons, which have a negative electrical charge; and neutrons, which have no charge. Protons and neutrons are packed into the nucleus, while electrons spin around outside. The number of protons is what gives an atom its chemical identity. An atom with one proton is an atom of hydrogen, one with two protons is helium, with three protons is lithium, and so on up the scale. Each time you add a proton you get a new element. The number of protons in an atom is always balanced by an equal number of electrons.
 Neutrons don’t influence an atom’s identity, but they make its mass. The number of neutrons is generally about the same as the number of protons, but they can vary up and down slightly. Add a neutron or two and you get an isotope. And what is an isotope? Each of two or more forms of the same element that contain equal numbers of protons but different numbers of neutrons in their nuclei, and so differ in atomic mass but not in chemical properties. They are often radioactive forms of an element.
Neutrons don’t influence an atom’s identity, but they make its mass. The number of neutrons is generally about the same as the number of protons, but they can vary up and down slightly. Add a neutron or two and you get an isotope. And what is an isotope? Each of two or more forms of the same element that contain equal numbers of protons but different numbers of neutrons in their nuclei, and so differ in atomic mass but not in chemical properties. They are often radioactive forms of an element.
So, neutrons and protons occupy the atom’s nucleus. The nucleus of an atom is tiny – only one millionth of a billionth of the full volume of the atom – but fantastically dense, since it contains virtually all the atom’s mass. If an atom could be expanded to the size of the mosque in Mecca, the nucleus would be only about the size of a fly, but a fly many thousands of times heavier than the mosque. It was this spaciousness that made Rutherford wonder.
It is still a fairly astounding notion It is still an amazing idea to consider that atoms are mostly empty space, and that the solidity all around us is an illusion. When two objects come together in the real world, they don’t actually hit each other. Rather, the negatively charged fields of the two objects push each other away. When you sit in a chair, you’re not actually sitting there, but just above it at a height of one angstrom (a hundred millionth of a centimeter), your electrons and its electrons do not allow you to get closer than that.
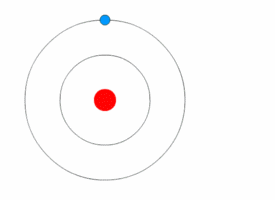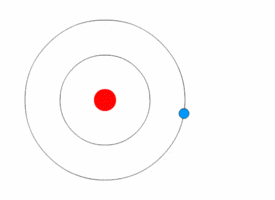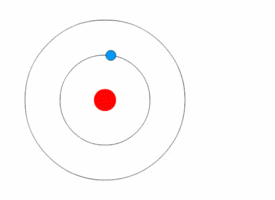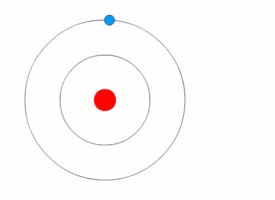
The picture that nearly everybody has in mind of an atom is of an electron or two flying around a nucleus, like planets orbiting a sun. It is completely wrong. In fact, as physicists were soon to realize, electrons are not like orbiting planets at all, but more like the blades of a fan, managing to fill every bit of space in their orbits simultaneously (but with the crucial difference that the blades of a fan only seem to be everywhere at once; electrons are).
Very little of this was understood in 1910 or for many years afterwards. Rutherford’s finding made some large and immediate problems, such as no electron should be able to orbit a nucleus without crashing. Electrodynamic theory demanded that a flying electron should very quickly run out of energy – in only a moment or so – and spiral into the nucleus, with disastrous results for both. There was also the problem of how protons with their positive charges could group together inside the nucleus without blowing themselves and the rest of the atom apart. Clearly whatever was going on down there in the world of the very small did not follow the same laws as in the macro world.
 As physicists began to explore this subatomic kingdom, they realized it wasn’t just different from anything we knew, but different from anything we imagined. So think how it must have felt to Rutherford and his colleagues in the early 1910s when it was all brand new.
As physicists began to explore this subatomic kingdom, they realized it wasn’t just different from anything we knew, but different from anything we imagined. So think how it must have felt to Rutherford and his colleagues in the early 1910s when it was all brand new.
One of the people working with Rutherford was a likable young Dane named Niels Bohr. In 1913, as he was puzzling over the structure of the atom, Bohr had an idea so exciting that he cancelled his honeymoon to write a paper on it. Because physicists couldn’t see anything so small as an atom, they had to try to work out its structure from how it behaved when they did things to it, as Rutherford had done by firing alpha particles at foil. One puzzle was spectrum readings of the wavelengths of hydrogen. These produced patterns showing that hydrogen atoms emitted energy at certain wavelengths but not others. It was rather like someone arriving at different places who was never seen traveling between them. No one could understand it.
Bohr explained how electrons could stop falling into the nucleus by suggesting they could move only in certain orbits. According to his theory, an electron moving between orbits would disappear from one and reappear instantly in another without visiting the space between. This idea – the famous “quantum leap” – is of course strange, but it was too good not to be true. It not only kept electrons from spiraling into the nucleus; it also explained hydrogen’s confusing wavelengths. The electrons only appeared in certain orbits because they only existed in certain orbits. It was an amazing insight, and it won Bohr the 1922 Nobel Prize in physics, the year after Einstein received his.
 Meanwhile Rutherford came up with a model that explained why the nuclei didn’t blow up. He saw that they must be offset by some type of neutralizing particles, which he called neutrons. The idea was simple and appealing, but not easy to prove. Rutherford’s associate, James Chadwick, gave eleven years to hunting for neutrons before finally succeeding in 1932. He, too, got a Nobel Prize in physics, in 1935. Understanding the neutron was necessary for the development of the atomic bomb. Because neutrons have no charge, they aren’t pushed away by electrical fields at the heart of an atom and so could be fired like tiny torpedoes into an atomic nucleus, setting off the destructive process known as fission.
Meanwhile Rutherford came up with a model that explained why the nuclei didn’t blow up. He saw that they must be offset by some type of neutralizing particles, which he called neutrons. The idea was simple and appealing, but not easy to prove. Rutherford’s associate, James Chadwick, gave eleven years to hunting for neutrons before finally succeeding in 1932. He, too, got a Nobel Prize in physics, in 1935. Understanding the neutron was necessary for the development of the atomic bomb. Because neutrons have no charge, they aren’t pushed away by electrical fields at the heart of an atom and so could be fired like tiny torpedoes into an atomic nucleus, setting off the destructive process known as fission.
Everyone now was trying to understand the strange behaviour of electrons. The main problem was that the electron sometimes behaved like a particle and sometimes like a wave. This impossible double nature drove physicists nearly mad. For the next decade, they thought and offered competing hypotheses. In France, they found that some oddities in the behavior of electrons disappeared when we regarded them as waves. This excited an Austrian, Erwin Schrödinger, who created a useful system called wave mechanics. At almost the same time the German Werner Heisenberg had a competing theory called matrix mechanics. This was so mathematically complex that no-one really understood it, including Heisenberg (“I do not even know what a matrix is,” Heisenberg despaired to a friend at one point), but it seemed to solve problems that Schrödinger’s waves failed to explain. The result was that physics had two theories that produced the same results.
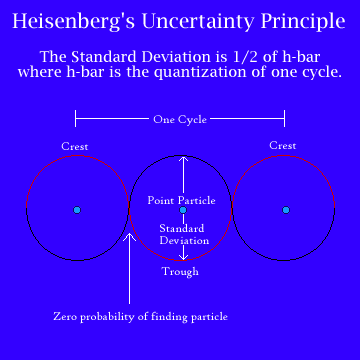 Finally, in 1926, Heisenberg came up with a compromise, making a new subject that came to be known as quantum mechanics. At its heart was Heisenberg’s Uncertainty Principle, which states that the electron is a particle but a particle that can be described in terms of waves. The uncertainty is that we can know the path an electron takes as it moves through a space or we can know where it is at any moment, but we cannot know both. Trying to measure one must disturb the other.
Finally, in 1926, Heisenberg came up with a compromise, making a new subject that came to be known as quantum mechanics. At its heart was Heisenberg’s Uncertainty Principle, which states that the electron is a particle but a particle that can be described in terms of waves. The uncertainty is that we can know the path an electron takes as it moves through a space or we can know where it is at any moment, but we cannot know both. Trying to measure one must disturb the other.
What this means is that you can never predict where an electron will be. You can only suggest the probability of it being there. In a sense, an electron doesn’t exist until it is observed. Or, put slightly differently, until it is observed an electron must be at once everywhere and nowhere.
If this is confusing, don’t worry! It was also confusing to physicists, too.
So the atom was unlike the image most people had created. The electron doesn’t fly around the nucleus like a planet around its sun, but instead is more like a cloud. The “shell” of an atom isn’t hard casing, as diagrams sometimes show it, but simply fuzzy electron clouds. The cloud itself is just a zone of statistical probability showing the area where the electron stays. Thus an atom, if you could see it, would look more like a very fuzzy tennis ball than a hard-edged metallic sphere.
It seemed as if there was no end of strangeness. For the first time, scientists had seen an area of the universe that our brains just aren’t capable of understanding. As physicists explored more deeply, they realized they had found a world where not only could electrons jump from one orbit to another without traveling across any intervening space, but matter could pop into existence from nothing at all – provided it disappears again very fast.

Schrödinger, our friend who invented wave mechanics, offered a famous thought experiment in which an imaginary cat was put in a box with one atom of a radioactive substance on a bottle of hydrocyanic acid. If the particle degraded within an hour, a mechanism would break the bottle and poison the cat. If not, the cat would live. But we could not know which was true, so there was no choice, scientifically, but to see the cat as 100% alive and 100% dead at the same time. This means, as Stephen Hawking said, that we cannot “predict future events exactly if one cannot even measure the present state of the universe precisely!”
Because of its oddities, many physicists disliked quantum theory, especially Einstein, who couldn’t bear the idea of a universe where some things were forever unknowable. Also, the idea of action at a distance – that one particle could influence another trillions of miles away – disobeyed the theory of relativity, which said nothing could outrace the speed of light. No-one, incidentally, has ever explained how. Scientists have dealt with this problem by not thinking about it.
 Above all, quantum physics introduced an untidiness that hadn’t existed before. Suddenly you needed two sets of laws to explain the behaviour of the universe – quantum theory for the world of the very small and relativity theory for the larger universe. The gravity of relativity was brilliant at explaining why planets orbited suns or why galaxies tended to gather together, but had no influence at particle level. To explain what kept atoms together, other forces were needed, and in the 1930s two were discovered: the strong nuclear force and weak nuclear force.
Above all, quantum physics introduced an untidiness that hadn’t existed before. Suddenly you needed two sets of laws to explain the behaviour of the universe – quantum theory for the world of the very small and relativity theory for the larger universe. The gravity of relativity was brilliant at explaining why planets orbited suns or why galaxies tended to gather together, but had no influence at particle level. To explain what kept atoms together, other forces were needed, and in the 1930s two were discovered: the strong nuclear force and weak nuclear force.
The weak nuclear force, despite its name, is ten billion billion billion times stronger than gravity, and the strong nuclear force is even more powerful – vastly, in fact – but their influence covers only the tiniest distances. The strong force reaches out only to about 1/100,000 of the diameter of an atom. That’s why the nuclei of atoms are so dense and why elements with big, crowded nuclei are so unstable: the strong force just can’t hold on to all the protons.
Einstein disliked the idea that the universe had two sets of laws, too. He spent the rest of his life searching for a way to tie these together, and always failed. His colleagues thought, and still think, that he wasted the second half of his life.
If you want to watch some videos on this topic, you can click on the links to YouTube videos below.
If you want to answer questions on this article to test how much you understand, you can click on the green box: Finished Reading?
Videos :
4. Dalton’s Atomic Theory (7:00)
5. Dalton’s Atomic Theory 2 (4:00)
6. Rutherford’s Atomic Model (4:00)
7. Rutherford’s atomic Model 2 (4:00)
8. Rutherford’s Gold Foil Experiment (4:00)
9. The history of Atomic Chemistry (10:00)
10. Thomson's Model of an Atom (3:00)
12. Bohr’s model of an Atom (5:00)
13. Chadwick and the Neutron (6:00)
