Course description
The Discovery of Elements & their Classification

In the early 1800s in England, there was a new fashion: inhaling nitrous oxide, or laughing gas. For the next half century it would be the drug of choice for young people. Theaters put on ‘laughing gas evenings’ where volunteers could get a thrill free of charge by inhaling the gas and then entertaining the audience by laughing, falling over and so on. It was the early 19th century version of crazy YouTube videos. It wasn’t until 1846 that anyone found a practical use for nitrous oxide, as an anesthetic.
I mention this to show that chemistry, so inventive in the 18th century with Lavoisier and Priestley, lost its way in the first decades of the nineteenth, much like geology would in the early twentieth. Partly it was to do with lack of equipment and partly it was social. Chemistry was, generally speaking, a science for businesspeople, for those who worked with coal, minerals and dyes, and not gentlemen, who were attracted to geology, natural history, and physics.
But then along came an unlikely character named Count von Rumford, who, despite this title, began life in Massachusetts in 1753 as Benjamin Thompson. Thompson was ambitious, handsome, occasionally courageous and extremely clever, but not very worried by morals. At nineteen, he married a rich widow fourteen years his senior, but, during The American War of Independence, he unwisely sided with the British, and, in 1776, facing arrest, he left his wife and child and escaped just ahead of a mob of rebels. He went to Germany, where he was military advisor to the government of Bavaria (in southern Germany). He so impressed the authorities that in 1791 he was named Count von Rumford.

In between all these activities, he somehow found time to do a good deal of solid science. He became the world authority on thermodynamics and the first to explain the principles of the convection of fluids and the circulation of ocean currents. He also invented several useful objects, including a drip coffeemaker and thermal underwear.
But why am I talking about Rumford? Well, in 1799, during a brief stay in London, he founded the Royal Institution, another of the many academic societies starting all over Britain in the late 18th and early 19th centuries. For a time it was almost the only institution to promote the young science of chemistry, thanks almost entirely to a brilliant young man, named Humphry Davy, who was appointed the institution’s first professor of chemistry.
Humphrydavy.jpg (200×270)Soon afterwards, Davy began to identify new elements one after another – potassium, sodium, magnesium, calcium, strontium, and aluminum or aluminium. He discovered so many elements because he developed a brilliant technique for applying electricity to a molten substance – electrolysis, as it is known. Altogether he discovered a dozen elements, a fifth of the known total of his day. Davy might have achieved more, but, unfortunately, as a young man he grew to appreciate the pleasures of nitrous oxide. Eventually, in 1829, it killed him.
Fortunately more sober types were at work elsewhere. In 1811, an Italian with the wonderful name of Lorenzo Romano Amadeo Carlo Avogadro, Count of Quarequa and Cerreto, made a discovery that proved highly significant in the long term – namely, that two equal volumes of gases of any type, if kept at the same pressure and temperature, will contain identical numbers of molecules.
Two things were notable about Avogadro’s Principle. First, it provided a basis for measuring the size and weight of atoms. Using Avogadro’s mathematics, chemists could work out, for instance, that a typical atom had a diameter of 0.00000008 centimeters, which is very little indeed. And second, almost no-one knew about Avogadro’s principle for almost fifty years.
 Partly this was because Avogadro worked alone, communicated very little with other scientists, published very few research papers, and attended no meetings. But also it was because there were no meetings to attend and few chemical journals to publish in. This was extraordinary. The Industrial Revolution was based on developments in chemistry, and yet as an organized science chemistry barely existed for decades.
There was no regular journal until 1848, by which time most learned societies in Britain – Geological, Geographical, Zoological, Horticultural, and Linnaean (for naturalists and botanists) – were at least twenty years old and often much more. Because chemistry was so slow to get organized, news of Avogadro’s important breakthrough of 1811 didn’t spread until the first international chemistry meeting of scientists in 1860.
So, the first few years of the 19th century gave us twelve new elements and Avogadro’s ground-breaking work on the size of molecules but these did not help to create a unified chemistry. Because chemists for so long worked in isolation, standards were slow to develop. For instance, until well into the second half of the century, the formula H2O2 might mean water to one chemist but hydrogen peroxide to another. There was not one molecule that was represented the same way everywhere.
Chemists also used a really confusing variety of symbols and abbreviations, often self-invented. Sweden’s J. J. Berzelius brought much-needed order by abbreviating the elements on the basis of their Greek or Latin names, which is why the abbreviation for iron is Fe (from the Latin ferrum) and for silver is Ag (from the Latin argentum). To show the number of atoms in a molecule, Berzelius used a superscript (up in the air) notation, as in H2O. Later, for no special reason, it became a subscript: H2O.
Partly this was because Avogadro worked alone, communicated very little with other scientists, published very few research papers, and attended no meetings. But also it was because there were no meetings to attend and few chemical journals to publish in. This was extraordinary. The Industrial Revolution was based on developments in chemistry, and yet as an organized science chemistry barely existed for decades.
There was no regular journal until 1848, by which time most learned societies in Britain – Geological, Geographical, Zoological, Horticultural, and Linnaean (for naturalists and botanists) – were at least twenty years old and often much more. Because chemistry was so slow to get organized, news of Avogadro’s important breakthrough of 1811 didn’t spread until the first international chemistry meeting of scientists in 1860.
So, the first few years of the 19th century gave us twelve new elements and Avogadro’s ground-breaking work on the size of molecules but these did not help to create a unified chemistry. Because chemists for so long worked in isolation, standards were slow to develop. For instance, until well into the second half of the century, the formula H2O2 might mean water to one chemist but hydrogen peroxide to another. There was not one molecule that was represented the same way everywhere.
Chemists also used a really confusing variety of symbols and abbreviations, often self-invented. Sweden’s J. J. Berzelius brought much-needed order by abbreviating the elements on the basis of their Greek or Latin names, which is why the abbreviation for iron is Fe (from the Latin ferrum) and for silver is Ag (from the Latin argentum). To show the number of atoms in a molecule, Berzelius used a superscript (up in the air) notation, as in H2O. Later, for no special reason, it became a subscript: H2O.  Despite occasional tidying, chemistry by the second half of the 19th century was in a mess, which is why everybody was so pleased by the arrival in 1869 of an odd and crazed-looking professor at the University of St. Petersburg, named Dmitri Ivanovich Mendeleyev.
Mendeleyev was born in 1834 in the far west of Siberia, into a well-educated, quite wealthy and very large family – so large, in fact, that history has lost track of exactly how many Mendeleyevs there were: some say there were fourteen children, some seventeen. All agree, at any rate, that Dmitri was the youngest. Luck was not always with the Mendeleyevs. When Dmitri was small his father, the headmaster of a local school, went blind and his mother had to go out to work. Clearly an extraordinary woman, she eventually became the manager of a successful glass factory. All went well until 1848, when the factory burned down and the family fell into poverty. Determined to get her youngest child an education, Mrs. Mendeleyev hitchhiked with young Dmitri four thousand miles to St. Petersburg and left him at the Institute of Pedagogy. Worn out, she died.
Mendeleyev completed his studies and got a position at the local university. There he was a competent but not outstanding chemist, known more for his wild hair and beard, which he had cut just once a year, than for his gifts in the laboratory.
However, in 1869, at the age of thirty-five, he began to play around with the idea of arranging the elements. At the time, elements were normally grouped in two ways, either by atomic weight (using Avogadro’s Principle) or by common properties (whether they were metals or gases, for instance). Mendeleyev’s breakthrough was to see that the two could be combined in a single table.
As is often the way in science, the principle was not actually new. Three years previously, an amateur chemist in England named John Newlands, suggested that when elements were arranged by weight they repeated certain properties (or characteristics) – in a way, to harmonize – at every eighth place on the scale. Newlands called it the Law of Octaves and compared the arrangement to the octaves on a piano. The idea was considered ridiculous and people refused to take it seriously. At meetings, comic members of the audience would sometimes ask him if he could get his elements to play them a little tune. Discouraged, Newlands gave up the idea and soon disappeared altogether.
Despite occasional tidying, chemistry by the second half of the 19th century was in a mess, which is why everybody was so pleased by the arrival in 1869 of an odd and crazed-looking professor at the University of St. Petersburg, named Dmitri Ivanovich Mendeleyev.
Mendeleyev was born in 1834 in the far west of Siberia, into a well-educated, quite wealthy and very large family – so large, in fact, that history has lost track of exactly how many Mendeleyevs there were: some say there were fourteen children, some seventeen. All agree, at any rate, that Dmitri was the youngest. Luck was not always with the Mendeleyevs. When Dmitri was small his father, the headmaster of a local school, went blind and his mother had to go out to work. Clearly an extraordinary woman, she eventually became the manager of a successful glass factory. All went well until 1848, when the factory burned down and the family fell into poverty. Determined to get her youngest child an education, Mrs. Mendeleyev hitchhiked with young Dmitri four thousand miles to St. Petersburg and left him at the Institute of Pedagogy. Worn out, she died.
Mendeleyev completed his studies and got a position at the local university. There he was a competent but not outstanding chemist, known more for his wild hair and beard, which he had cut just once a year, than for his gifts in the laboratory.
However, in 1869, at the age of thirty-five, he began to play around with the idea of arranging the elements. At the time, elements were normally grouped in two ways, either by atomic weight (using Avogadro’s Principle) or by common properties (whether they were metals or gases, for instance). Mendeleyev’s breakthrough was to see that the two could be combined in a single table.
As is often the way in science, the principle was not actually new. Three years previously, an amateur chemist in England named John Newlands, suggested that when elements were arranged by weight they repeated certain properties (or characteristics) – in a way, to harmonize – at every eighth place on the scale. Newlands called it the Law of Octaves and compared the arrangement to the octaves on a piano. The idea was considered ridiculous and people refused to take it seriously. At meetings, comic members of the audience would sometimes ask him if he could get his elements to play them a little tune. Discouraged, Newlands gave up the idea and soon disappeared altogether.  Mendeleyev used a slightly different approach, placing his elements into groups of seven, but employed fundamentally the same principle. Suddenly the idea seemed brilliant. Because the properties repeated themselves periodically, the invention became known as the periodic table.
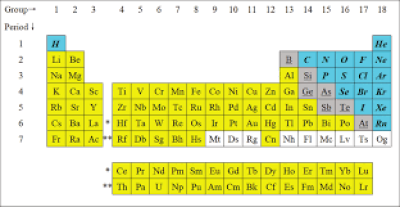
Mendeleyev got the idea for his table from the card game known as patience, where cards are arranged by suit (clubs, diamonds, hearts and spades) horizontally and by number vertically. Using a similar concept, he arranged the elements in horizontal rows called periods and vertical columns called groups. This instantly showed one set of relationships when read up and down and another when read from side to side. Specifically, the vertical columns put together chemicals that have similar properties. So, copper sits on top of silver and silver sits on top of gold because of their chemical similarities as metals, while helium, neon, and argon are in a column made up of gases. The horizontal rows, meanwhile, arrange the chemicals in ascending order by the number of protons in their nuclei, or their atomic number.
The organizing principle is like this: hydrogen has just one proton, and so it has an atomic number of one and comes first on the chart; uranium has ninety-two protons, and so it comes near the end and has an atomic number of ninety-two. In this sense, chemistry really is just a matter of counting. (Atomic number, incidentally, is not to be confused with atomic weight, which is the number of protons plus the number of neutrons in a given element.)
There was still a great deal that wasn’t known or understood. Hydrogen is the most common element in the universe, and yet no one would guess that for another thirty years. Helium, the second most common element, had only been found the year before and not on Earth but in the Sun. It wouldn’t be isolated until 1895. Even so, thanks to Mendeleyev’s invention, chemistry was now on a firm footing.
For chemists, the Periodic Table established an immediate and clear order and was widely respected and admired.
Today we have 120 or so known elements – ninety-two natural ones plus a couple of dozen that have been created in labs. The actual number is unclear because the heavy, synthesized elements exist for only millionths of seconds and chemists sometimes argue over whether they have really been detected or not. In Mendeleyev’s day, just sixty-three elements were known, but part of his cleverness was to realize that the elements then known didn’t make a complete picture, that many pieces were missing. His table predicted, with pleasing accuracy, where we could insert new elements when they were found.
No-one knows, incidentally, how high the number of elements might go, though anything beyond an atomic weight of 168 can only be theoretical, but what is sure is anything that is found will fit neatly into Mendeleyev’s great scheme.
Mendeleyev used a slightly different approach, placing his elements into groups of seven, but employed fundamentally the same principle. Suddenly the idea seemed brilliant. Because the properties repeated themselves periodically, the invention became known as the periodic table.
Mendeleyev got the idea for his table from the card game known as patience, where cards are arranged by suit (clubs, diamonds, hearts and spades) horizontally and by number vertically. Using a similar concept, he arranged the elements in horizontal rows called periods and vertical columns called groups. This instantly showed one set of relationships when read up and down and another when read from side to side. Specifically, the vertical columns put together chemicals that have similar properties. So, copper sits on top of silver and silver sits on top of gold because of their chemical similarities as metals, while helium, neon, and argon are in a column made up of gases. The horizontal rows, meanwhile, arrange the chemicals in ascending order by the number of protons in their nuclei, or their atomic number.
The organizing principle is like this: hydrogen has just one proton, and so it has an atomic number of one and comes first on the chart; uranium has ninety-two protons, and so it comes near the end and has an atomic number of ninety-two. In this sense, chemistry really is just a matter of counting. (Atomic number, incidentally, is not to be confused with atomic weight, which is the number of protons plus the number of neutrons in a given element.)
There was still a great deal that wasn’t known or understood. Hydrogen is the most common element in the universe, and yet no one would guess that for another thirty years. Helium, the second most common element, had only been found the year before and not on Earth but in the Sun. It wouldn’t be isolated until 1895. Even so, thanks to Mendeleyev’s invention, chemistry was now on a firm footing.
For chemists, the Periodic Table established an immediate and clear order and was widely respected and admired.
Today we have 120 or so known elements – ninety-two natural ones plus a couple of dozen that have been created in labs. The actual number is unclear because the heavy, synthesized elements exist for only millionths of seconds and chemists sometimes argue over whether they have really been detected or not. In Mendeleyev’s day, just sixty-three elements were known, but part of his cleverness was to realize that the elements then known didn’t make a complete picture, that many pieces were missing. His table predicted, with pleasing accuracy, where we could insert new elements when they were found.
No-one knows, incidentally, how high the number of elements might go, though anything beyond an atomic weight of 168 can only be theoretical, but what is sure is anything that is found will fit neatly into Mendeleyev’s great scheme.  The 19th century held one last great surprise for chemists. It began in 1896 when Henri Becquerel in Paris carelessly left a packet of uranium salts on a wrapped photographic plate in a drawer. When he took the plate out some time later, he was surprised to discover that the salts had burned an impression in it, just as if the plate had been exposed to light. The salts were emitting rays of some sort.
Considering the importance of what he had found, Becquerel did a very strange thing: he turned the matter over to a graduate student for investigation. Fortunately the student was a recent arrival from Poland named Marie Curie. Working with her new husband, Pierre, Curie found that certain kinds of rocks poured out constant and extraordinary amounts of energy, yet without diminishing in size or changing in any detectable way. What she and her husband couldn’t know—what no one could know until Einstein explained things the following decade—was that the rocks were converting mass into energy in an exceedingly efficient way. Marie Curie dubbed the effect “radioactivity.” You can read her story in another article!
If you want to watch some videos on this topic, you can click on the links to YouTube videos below.
If you want to answer questions on this article to test how much you understand, you can click on the green box: Finished Reading?
Videos :
The 19th century held one last great surprise for chemists. It began in 1896 when Henri Becquerel in Paris carelessly left a packet of uranium salts on a wrapped photographic plate in a drawer. When he took the plate out some time later, he was surprised to discover that the salts had burned an impression in it, just as if the plate had been exposed to light. The salts were emitting rays of some sort.
Considering the importance of what he had found, Becquerel did a very strange thing: he turned the matter over to a graduate student for investigation. Fortunately the student was a recent arrival from Poland named Marie Curie. Working with her new husband, Pierre, Curie found that certain kinds of rocks poured out constant and extraordinary amounts of energy, yet without diminishing in size or changing in any detectable way. What she and her husband couldn’t know—what no one could know until Einstein explained things the following decade—was that the rocks were converting mass into energy in an exceedingly efficient way. Marie Curie dubbed the effect “radioactivity.” You can read her story in another article!
If you want to watch some videos on this topic, you can click on the links to YouTube videos below.
If you want to answer questions on this article to test how much you understand, you can click on the green box: Finished Reading?
Videos :
1. Benjamin Thompson (14:00)
2. Thermodynamics (11:00)
3. Humphry Davy (11:00)
4. Avogadro (6:00)
5. Berzelius (2:22)
6. Dmitri Mendeleyev (4:00)
7. Periodic Table (4:25)
8. Newlands (3:00)
9. Radioactivity (2:00)
10. Radioactivity -Henri Becquerel, Marie & Pierre Curie (15:00)



 Partly this was because Avogadro worked alone, communicated very little with other scientists, published very few research papers, and attended no meetings. But also it was because there were no meetings to attend and few chemical journals to publish in. This was extraordinary. The Industrial Revolution was based on developments in chemistry, and yet as an organized science chemistry barely existed for decades.
There was no regular journal until 1848, by which time most learned societies in Britain – Geological, Geographical, Zoological, Horticultural, and Linnaean (for naturalists and botanists) – were at least twenty years old and often much more. Because chemistry was so slow to get organized, news of Avogadro’s important breakthrough of 1811 didn’t spread until the first international chemistry meeting of scientists in 1860.
So, the first few years of the 19th century gave us twelve new elements and Avogadro’s ground-breaking work on the size of molecules but these did not help to create a unified chemistry. Because chemists for so long worked in isolation, standards were slow to develop. For instance, until well into the second half of the century, the formula H2O2 might mean water to one chemist but hydrogen peroxide to another. There was not one molecule that was represented the same way everywhere.
Chemists also used a really confusing variety of symbols and abbreviations, often self-invented. Sweden’s J. J. Berzelius brought much-needed order by abbreviating the elements on the basis of their Greek or Latin names, which is why the abbreviation for iron is Fe (from the Latin ferrum) and for silver is Ag (from the Latin argentum). To show the number of atoms in a molecule, Berzelius used a superscript (up in the air) notation, as in H2O. Later, for no special reason, it became a subscript: H2O.
Partly this was because Avogadro worked alone, communicated very little with other scientists, published very few research papers, and attended no meetings. But also it was because there were no meetings to attend and few chemical journals to publish in. This was extraordinary. The Industrial Revolution was based on developments in chemistry, and yet as an organized science chemistry barely existed for decades.
There was no regular journal until 1848, by which time most learned societies in Britain – Geological, Geographical, Zoological, Horticultural, and Linnaean (for naturalists and botanists) – were at least twenty years old and often much more. Because chemistry was so slow to get organized, news of Avogadro’s important breakthrough of 1811 didn’t spread until the first international chemistry meeting of scientists in 1860.
So, the first few years of the 19th century gave us twelve new elements and Avogadro’s ground-breaking work on the size of molecules but these did not help to create a unified chemistry. Because chemists for so long worked in isolation, standards were slow to develop. For instance, until well into the second half of the century, the formula H2O2 might mean water to one chemist but hydrogen peroxide to another. There was not one molecule that was represented the same way everywhere.
Chemists also used a really confusing variety of symbols and abbreviations, often self-invented. Sweden’s J. J. Berzelius brought much-needed order by abbreviating the elements on the basis of their Greek or Latin names, which is why the abbreviation for iron is Fe (from the Latin ferrum) and for silver is Ag (from the Latin argentum). To show the number of atoms in a molecule, Berzelius used a superscript (up in the air) notation, as in H2O. Later, for no special reason, it became a subscript: H2O.  Despite occasional tidying, chemistry by the second half of the 19th century was in a mess, which is why everybody was so pleased by the arrival in 1869 of an odd and crazed-looking professor at the University of St. Petersburg, named Dmitri Ivanovich Mendeleyev.
Mendeleyev was born in 1834 in the far west of Siberia, into a well-educated, quite wealthy and very large family – so large, in fact, that history has lost track of exactly how many Mendeleyevs there were: some say there were fourteen children, some seventeen. All agree, at any rate, that Dmitri was the youngest. Luck was not always with the Mendeleyevs. When Dmitri was small his father, the headmaster of a local school, went blind and his mother had to go out to work. Clearly an extraordinary woman, she eventually became the manager of a successful glass factory. All went well until 1848, when the factory burned down and the family fell into poverty. Determined to get her youngest child an education, Mrs. Mendeleyev hitchhiked with young Dmitri four thousand miles to St. Petersburg and left him at the Institute of Pedagogy. Worn out, she died.
Mendeleyev completed his studies and got a position at the local university. There he was a competent but not outstanding chemist, known more for his wild hair and beard, which he had cut just once a year, than for his gifts in the laboratory.
However, in 1869, at the age of thirty-five, he began to play around with the idea of arranging the elements. At the time, elements were normally grouped in two ways, either by atomic weight (using Avogadro’s Principle) or by common properties (whether they were metals or gases, for instance). Mendeleyev’s breakthrough was to see that the two could be combined in a single table.
As is often the way in science, the principle was not actually new. Three years previously, an amateur chemist in England named John Newlands, suggested that when elements were arranged by weight they repeated certain properties (or characteristics) – in a way, to harmonize – at every eighth place on the scale. Newlands called it the Law of Octaves and compared the arrangement to the octaves on a piano. The idea was considered ridiculous and people refused to take it seriously. At meetings, comic members of the audience would sometimes ask him if he could get his elements to play them a little tune. Discouraged, Newlands gave up the idea and soon disappeared altogether.
Despite occasional tidying, chemistry by the second half of the 19th century was in a mess, which is why everybody was so pleased by the arrival in 1869 of an odd and crazed-looking professor at the University of St. Petersburg, named Dmitri Ivanovich Mendeleyev.
Mendeleyev was born in 1834 in the far west of Siberia, into a well-educated, quite wealthy and very large family – so large, in fact, that history has lost track of exactly how many Mendeleyevs there were: some say there were fourteen children, some seventeen. All agree, at any rate, that Dmitri was the youngest. Luck was not always with the Mendeleyevs. When Dmitri was small his father, the headmaster of a local school, went blind and his mother had to go out to work. Clearly an extraordinary woman, she eventually became the manager of a successful glass factory. All went well until 1848, when the factory burned down and the family fell into poverty. Determined to get her youngest child an education, Mrs. Mendeleyev hitchhiked with young Dmitri four thousand miles to St. Petersburg and left him at the Institute of Pedagogy. Worn out, she died.
Mendeleyev completed his studies and got a position at the local university. There he was a competent but not outstanding chemist, known more for his wild hair and beard, which he had cut just once a year, than for his gifts in the laboratory.
However, in 1869, at the age of thirty-five, he began to play around with the idea of arranging the elements. At the time, elements were normally grouped in two ways, either by atomic weight (using Avogadro’s Principle) or by common properties (whether they were metals or gases, for instance). Mendeleyev’s breakthrough was to see that the two could be combined in a single table.
As is often the way in science, the principle was not actually new. Three years previously, an amateur chemist in England named John Newlands, suggested that when elements were arranged by weight they repeated certain properties (or characteristics) – in a way, to harmonize – at every eighth place on the scale. Newlands called it the Law of Octaves and compared the arrangement to the octaves on a piano. The idea was considered ridiculous and people refused to take it seriously. At meetings, comic members of the audience would sometimes ask him if he could get his elements to play them a little tune. Discouraged, Newlands gave up the idea and soon disappeared altogether.  Mendeleyev used a slightly different approach, placing his elements into groups of seven, but employed fundamentally the same principle. Suddenly the idea seemed brilliant. Because the properties repeated themselves periodically, the invention became known as the periodic table.
Mendeleyev got the idea for his table from the card game known as patience, where cards are arranged by suit (clubs, diamonds, hearts and spades) horizontally and by number vertically. Using a similar concept, he arranged the elements in horizontal rows called periods and vertical columns called groups. This instantly showed one set of relationships when read up and down and another when read from side to side. Specifically, the vertical columns put together chemicals that have similar properties. So, copper sits on top of silver and silver sits on top of gold because of their chemical similarities as metals, while helium, neon, and argon are in a column made up of gases. The horizontal rows, meanwhile, arrange the chemicals in ascending order by the number of protons in their nuclei, or their atomic number.
The organizing principle is like this: hydrogen has just one proton, and so it has an atomic number of one and comes first on the chart; uranium has ninety-two protons, and so it comes near the end and has an atomic number of ninety-two. In this sense, chemistry really is just a matter of counting. (Atomic number, incidentally, is not to be confused with atomic weight, which is the number of protons plus the number of neutrons in a given element.)
There was still a great deal that wasn’t known or understood. Hydrogen is the most common element in the universe, and yet no one would guess that for another thirty years. Helium, the second most common element, had only been found the year before and not on Earth but in the Sun. It wouldn’t be isolated until 1895. Even so, thanks to Mendeleyev’s invention, chemistry was now on a firm footing.
For chemists, the Periodic Table established an immediate and clear order and was widely respected and admired.
Today we have 120 or so known elements – ninety-two natural ones plus a couple of dozen that have been created in labs. The actual number is unclear because the heavy, synthesized elements exist for only millionths of seconds and chemists sometimes argue over whether they have really been detected or not. In Mendeleyev’s day, just sixty-three elements were known, but part of his cleverness was to realize that the elements then known didn’t make a complete picture, that many pieces were missing. His table predicted, with pleasing accuracy, where we could insert new elements when they were found.
No-one knows, incidentally, how high the number of elements might go, though anything beyond an atomic weight of 168 can only be theoretical, but what is sure is anything that is found will fit neatly into Mendeleyev’s great scheme.
Mendeleyev used a slightly different approach, placing his elements into groups of seven, but employed fundamentally the same principle. Suddenly the idea seemed brilliant. Because the properties repeated themselves periodically, the invention became known as the periodic table.
Mendeleyev got the idea for his table from the card game known as patience, where cards are arranged by suit (clubs, diamonds, hearts and spades) horizontally and by number vertically. Using a similar concept, he arranged the elements in horizontal rows called periods and vertical columns called groups. This instantly showed one set of relationships when read up and down and another when read from side to side. Specifically, the vertical columns put together chemicals that have similar properties. So, copper sits on top of silver and silver sits on top of gold because of their chemical similarities as metals, while helium, neon, and argon are in a column made up of gases. The horizontal rows, meanwhile, arrange the chemicals in ascending order by the number of protons in their nuclei, or their atomic number.
The organizing principle is like this: hydrogen has just one proton, and so it has an atomic number of one and comes first on the chart; uranium has ninety-two protons, and so it comes near the end and has an atomic number of ninety-two. In this sense, chemistry really is just a matter of counting. (Atomic number, incidentally, is not to be confused with atomic weight, which is the number of protons plus the number of neutrons in a given element.)
There was still a great deal that wasn’t known or understood. Hydrogen is the most common element in the universe, and yet no one would guess that for another thirty years. Helium, the second most common element, had only been found the year before and not on Earth but in the Sun. It wouldn’t be isolated until 1895. Even so, thanks to Mendeleyev’s invention, chemistry was now on a firm footing.
For chemists, the Periodic Table established an immediate and clear order and was widely respected and admired.
Today we have 120 or so known elements – ninety-two natural ones plus a couple of dozen that have been created in labs. The actual number is unclear because the heavy, synthesized elements exist for only millionths of seconds and chemists sometimes argue over whether they have really been detected or not. In Mendeleyev’s day, just sixty-three elements were known, but part of his cleverness was to realize that the elements then known didn’t make a complete picture, that many pieces were missing. His table predicted, with pleasing accuracy, where we could insert new elements when they were found.
No-one knows, incidentally, how high the number of elements might go, though anything beyond an atomic weight of 168 can only be theoretical, but what is sure is anything that is found will fit neatly into Mendeleyev’s great scheme.  The 19th century held one last great surprise for chemists. It began in 1896 when Henri Becquerel in Paris carelessly left a packet of uranium salts on a wrapped photographic plate in a drawer. When he took the plate out some time later, he was surprised to discover that the salts had burned an impression in it, just as if the plate had been exposed to light. The salts were emitting rays of some sort.
Considering the importance of what he had found, Becquerel did a very strange thing: he turned the matter over to a graduate student for investigation. Fortunately the student was a recent arrival from Poland named Marie Curie. Working with her new husband, Pierre, Curie found that certain kinds of rocks poured out constant and extraordinary amounts of energy, yet without diminishing in size or changing in any detectable way. What she and her husband couldn’t know—what no one could know until Einstein explained things the following decade—was that the rocks were converting mass into energy in an exceedingly efficient way. Marie Curie dubbed the effect “radioactivity.” You can read her story in another article!
If you want to watch some videos on this topic, you can click on the links to YouTube videos below.
If you want to answer questions on this article to test how much you understand, you can click on the green box: Finished Reading?
Videos :
The 19th century held one last great surprise for chemists. It began in 1896 when Henri Becquerel in Paris carelessly left a packet of uranium salts on a wrapped photographic plate in a drawer. When he took the plate out some time later, he was surprised to discover that the salts had burned an impression in it, just as if the plate had been exposed to light. The salts were emitting rays of some sort.
Considering the importance of what he had found, Becquerel did a very strange thing: he turned the matter over to a graduate student for investigation. Fortunately the student was a recent arrival from Poland named Marie Curie. Working with her new husband, Pierre, Curie found that certain kinds of rocks poured out constant and extraordinary amounts of energy, yet without diminishing in size or changing in any detectable way. What she and her husband couldn’t know—what no one could know until Einstein explained things the following decade—was that the rocks were converting mass into energy in an exceedingly efficient way. Marie Curie dubbed the effect “radioactivity.” You can read her story in another article!
If you want to watch some videos on this topic, you can click on the links to YouTube videos below.
If you want to answer questions on this article to test how much you understand, you can click on the green box: Finished Reading?
Videos :