Course description
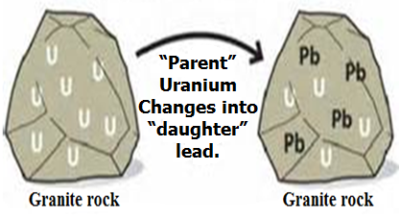
It is a condition established in a parent/daughter mixture when both parent and daughter are radioactive and when the daughter’s half-life is shorter than that of the parent. If the daughter’s half-life exceeds that of the parent, equilibrium will never be reached. Let N_1^0 and N_2^0 be the initial amount of parent and daughter substance respectively at t = 0. At any instant t, the amount of the parent substance be N1 and its decay constant is λ1, while N2 be the amount of the daughter at the same instant, t and λ2 its decay constant. Let dN1 be the number of atoms that decayed in the infinitesimal time interval dt. Then the rate at which the patent decays is (dN_1)/dt=-λ_1 (Activity of parent)
Since an atom of the daughter appears every time an atom of the parent disappears, the rate of formation of the daughter is . At the same time interval dt, the daughter is also disappearing according to its own decay constant and the rate of disappearance of the daughter is obviously λ_2 N_2 . So the rate of net increase (Fig. 7) of the daughter at the same time interval dt, is given by
where λ1 cannot be zero, so N1 must be constant.
Moreover, the amounts of the two substances present are inversely proportional to their decay constant. However, eqn. (vii) shows that the activity of the parent and activity of the daughter become equal, therefore equilibrium has been reached and the lines become parallel (Fig. 9 e). At this equilibrium mixture, the daughter appears to decay with the half-life of the parent. When the daughter is isolated from the mixture, it has its expected half-life. The simplest explanation for their appearing to be equal is that the daughter can’t decay until it is formed, and so the rate of formation of the daughter equals the rate of decay of the parent, which is very slow. Therefore, the parent and daughter appear to have the same half-lives, so that the decay rate of daughter will be very poor as the parent. Under these conditions the daughter breaks up as fast as it is formed to become very slow decay rate. For example, seven half-lives of 222Rn must elapse before the activity of the daughter (222Rn) equals 99% of the activity of the parent (226Ra) (Fig. 9 c).
If the half-life of the parent is less than that of the daughter, then a constant relationship is not achieved. Instead, the activity of the daughter increases initially, reaches a maximum, and then decreases with a half-life intermediate between the half-lives of the parent and the daughter.