Course description
Radioactivity & Radiation:
If a nucleus be too heavy or has excess energy, and not has the right balance of protons and neutrons then it will be a unstable nucleus. To be stable it will emit radiation (particles of matter and rays of energy, Fig. 1a), and in so doing becomes nucleus of other elements (Fig. 1b). The process of emitting radiation is called radioactivity. Despite the strength of the forces that hold nucleons together to form an atomic nucleus, many nuclides are unstable and spontaneously change into other nuclides by radioactive decay, actually, they don’t have enough binding energy to hold the nucleus together due to an excess of either protons or neutrons. However, radioactivity is a spontaneous and self-disruptive activity exhibited almost entirely by some heavy elements of atomic weights greater than about 206 (Z >82), occurring in nature. Elements in which radioactivity takes place are called radioactive elements. The particles and energy given off by these elements are formed of nuclear radiation. In making the emissions, the nucleus of a radioactive atom is said to decay. The radiation of a radioactive substance is harmful to life. Properly used, however, this radiation is extremely useful in science, medicine (Fig. 1c), agriculture, and industry.
The radioactivity of an element arises from the radioactivity of one or more of its isotopes. Most elements in nature have no radioactive isotopes, although such isotopes can be prepared artificially.
Radioactive elements in nature can be placed in three general categories:
(i) Cosmogonic – formed as a result of cosmic ray interactions (14C, 7Be, 3H, etc.).
(ii) Primordial – from before the creation of the Earth (238U, 232Th, 40K, etc.).
(iii) Human produced – enhanced or formed due to human actions (minor amounts compared to natural) (3H 137Cs, 134Cs, 131I, 90Sr).
Radionuclides are found naturally in air, water and soil. They are even found in our body. Every day, we ingest and inhale radionuclides in our air and food and the water. Natural radioactivity is common in the rocks and soil that makes up our planet, in water and oceans, and in our building materials and homes. At naturally occurring intensities, radioactivity is undetectable by unaided human senses. It could not have been detected without the photographic technique, i.e., earlier than the end of the 19th century.
Rutherford and his coworkers first distinguished three components in the radiations from radionulides. These components were called alpha (42He nuclei), beta (electrons) and gamma (high-energy photons) (Fig. 1 d-f). Later, positron emission and electron capture were added to the list of decay modes (Fig. 2).
Fig. 2
Although most of the transformations release energy in the form of mass, they can also leave the remaining nucleus in an “excited” state, where it has more than its minimum amount of energy. It will therefore lose this extra energy by emitting a gamma ray — a very high frequency form of electromagnetic radiation. Gamma rays have no weight, and travel at the speed of light.
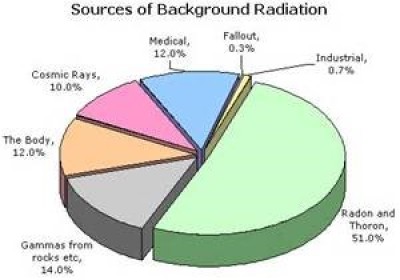
Fig. 3 Involvement of background radiation in %.
Units: The original unit for measuring the amount of radioactivity was the curie (Ci)–first defined to correspond to one gram of radium-226 and more recently defined as:
1 curie = 3.7x1010 radioactive decays per second [exactly].
In the International System of Units (SI) the curie has been replaced by the becquerel (Bq), where
1 becquerel = 1 radioactive decay per second = 2.703x10-11 Ci.
Conversion factors:
1 Ci = 3.7×1010 Bq = 37 GBq ; 1 μCi = 37,000 Bq = 37 kBq
1 Bq = 2.7×10−11 Ci = 2.7×10−5 µCi ; 1 GBq = 0.027 Ci