Course description
Different states of matter:
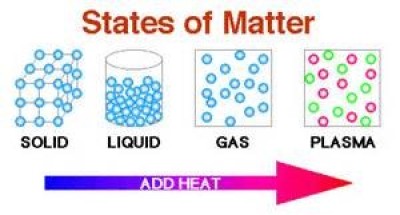
Fig. 1
Solid, liquid, gaseous and plasma are the four states for a given substance that can exist (Fig. 1). The chemical composition of the substance is the same in solid, liquid, and gaseous states. Properties of matter that can be changed with changing the state of a substance: density, elasticity, surface tension, viscosity, specific heat, conductivity, semi-conductivity, Ferro-magnetism, Para magnetism, reflecting properties of light etc.
Solid state: Atoms are closely packed, density is high and hence difficult to compress. Solids have a definite shape and volume at a given temperature. There are two types of solids i) crystalline and ii) non-crystalline or amorphous solids (Fig. 2). In crystalline solid atoms are arranged in a regular three-dimensional array with different types of packing. Crystalline solids are symmetrical and more rigid. It has a sharp melting point and characteristic geometrical shape. On the other hand, amorphous solids melt slowly. Amorphous solids are unsymmetrical and less rigid. Amorphous solids do not have definite geometrical shape.
Fig. 2
Liquid state: In the liquid state molecules are loosely packed compare to solid state and structural pattern of molecules is disordered and changing continually (Fig. 3). Density and viscosity are higher than gaseous state. Rigidity is low.
Fig. 3 Fig. 4 Fig.5
Gaseous state: Molecules are far apart and they are not arranged in a regular pattern (Fig. 4). Density and viscosity is low and hence highly compressible. It has no rigidity.
Plasma state: The fourth state of matter is plasma. Plasma is an ionized gas, a gas into which sufficient energy is provided to free electrons from atoms or molecules and to allow both species, ions and electrons, to coexist (Fig. 5). In effect a plasma is a cloud of protons, neutrons and electrons where all the electrons have come loose from their respective molecules and atoms. Plasmas are the most common state of matter in the universe comprising more than 99% of our visible universe and most of that not visible. Plasma occurs naturally and makes up the stuff of our sun, the core of stars (Fig. 6a) and occurs in quasars, x-ray beam emitting pulsars, and supernovas. On earth, plasma is naturally occurring in flames, lightning and the auroras. Most space plasmas have a very low density. Lightning (Fig. 6b), electric sparks, fluorescent lights, neon lights, plasma televisions,
(a) Fig. 6 (b)
Ductility is the property of certain materials to fail only after large stresses and strains have occurred.
Malleability is a physical property of metals that defines the ability to be hammered, pressed or rolled into thin sheets without breaking.